
Antiviral and Antivirulence Drugs
Prof Dr Martin Empting
Work in the Empting lab focuses on tackling innovative and difficult-to-address anti-infective targets such as bacterial virulence regulatory systems as well as un(der)explored anti-herpesviral persitance mediators. By this, we aspire to circumvent common resistance mechanisms and to refill the dried out development pipeline.
Our research and approach
We progress our antiinfective projects through all the stages of drug discovery. Our hit-finding strategies are selected individually for the target molecule at hand. In the case of receptors and enzymes with pockets druggable with small molecules (e.g. PqsR, PqsE), we employ fragment-based methodologies and transform identified starting points (hits) into drug-like molecules (leads) by medicinal chemistry optimization. In this regard, we follow a multi-parameter optimization considering not only potency but also pharmacokinetic and safety pharmacology endpoints. Ideally, leads are optimized towards preclinical profiling candidate status in order to facilitate preclinical development.
Difficult-to-drug macromolecule-macromolecule interactions provide challenging, yet, promising and notoriously underexploited points of disease intervention. We have been successful in applying above-mentioned fragment-based hit identification to a protein-DNA interaction essential for the lifecycle of human herpesvirus 8 (Kaposi´s sarcoma herpesvirus, KSHV) mediated by the latency-associated nuclear antigen (LANA). In order to supplement and complement these small molecule-directed approaches, we also apply the phage display technology for the screening of very short macrocyclic (constrained) peptide sequences. The resulting hits are readily synthetically accessible starting points amenable to medicinal chemistry optimization towards novel drug molecules at the boarders of Lipinski´s rule of five (or just slightly beyond). In the future, we aim to combine fragment- and phage-display-based approaches to generate peptide-small molecules hybrids with the aim to explore currently untapped chemical space.
Team members

Prof Dr Martin Empting
Group Leader

Eva-Maria Schönborn
PhD Student

Huilin Zhan
PhD Student

Konrad Wagner
PhD Student

Kyana Mazlom
PhD Student

Marces Calvin-Brown
PhD Student
Research projects
Targeting bacterial Pathogenicity (Pathoblockers)
With the aim to devise novel treatment modalities and to divert from classical bactericidal or bacteriostatic antibiotics, we design and optimize synthetic molecules, which specifically block bacterial pathogenicity traits. In this regard, we address transcriptional and post-transcriptional regulators of bacterial virulence. By this means, we interfere with a multitude of virulence factors and do not interfere with bacterial viability. The overall aim of the project is to discover and advance compounds for the treatment of Pseudomonas aeruginosa infections, which is a problematic ESKAPE pathogen and the major cause of morbidity and mortality in patients with cystic fibrosis. To this end, we address to regulator systems: A) the Pseudomonas quinolone signal (PQS) quorum sensing (QS) system and B) the carbon storage regulator (Csr; also called regulator of secondary metabolites, Rsm) system.
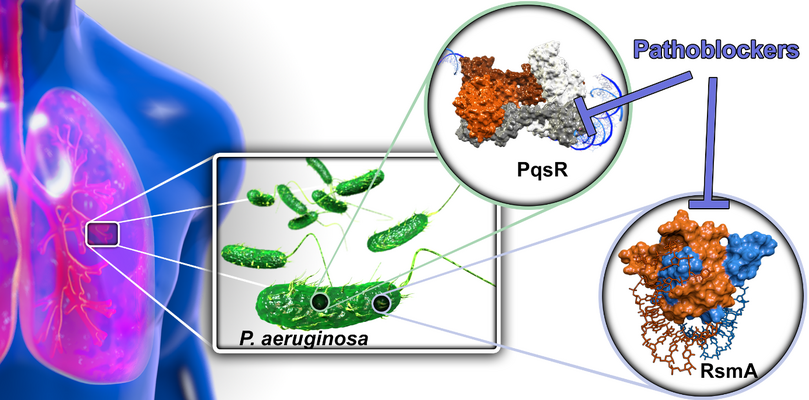
Pathoblockes
A) QS provides a way for bacteria to communicate with each other via small diffusible signal molecules. This ability is essential for population-wide coordination of infection processes. We target the signal molecule receptor and transcriptional regulator PqsR (MvfR) by compounds acting as inverse agonists. The PQS QS system is quite unique resulting in a pathogen-specific mode-of-action, which preserves the commensal (beneficial) microbiota. By means, we successfully achieved lead status with three chemical classes of pathoblockers showing promising potency in the nanomolar range combined with a suitable pharmacokinetic and in vitro safety pharmacology profile. This project is currently in the late lead optimization stage.
B) In contrast to the PQS QS, the Csr/Rsm system is astonishingly widespread among pathogenic bacteria. Hence, this higher order regulatory system holds promise as a potential broad-spectrum pathoblocker target. We were able to identify identify natural product-derived small molecule-like hits as well as novel peptidic macrocyclic starting points for medicinal chemistry optimization. Demonstration of cellular activity mediated by blocking this post-transcriptional regulator is the next step in this project.
Targeting persistence of human herpes viruses
The latency-associated nuclear antigen (LANA) is required for latent replication and persistence of Kaposi’sarcoma-associated herpesvirus/human herpesvirus 8. LANA exerts important functions in the host cell like cell survival, transcriptional control, latent viral DNA replication, and stable episome segregation during mitosis. It acts via tethering the virus episome to the host chromatin. We aim to discover novel antiviral drugs, which to inhibit the interaction between LANA and the viral genome. By this means, we should be able to disrupt the latent replication of KSHV.
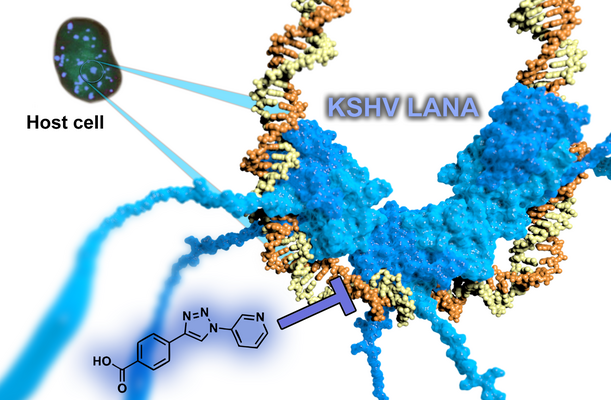
Replication of KSHV
We were successful in finding the first LANA inhibitors by a fragment-based approach. The resulting optimized hit provides an ideal starting point for subsequent hit-to-lead campaigns providing evident target-binding, suitable ligand efficiencies, and favourable physicochemical properties. Further improved compounds are currently underway with a view to demonstrate antiviral activities in cell-based assays.
